GenAI-Powered Translation for Pharma and Life Sciences
Posted on : October 23rd 2024
The pharmaceutical and life sciences industry is one of the most regulated sectors, and translation plays a critical role in ensuring that drugs, treatments, and patient health information are accurately conveyed across different languages and regions. And, whether it’s clinical trial data, regulatory submissions, or patient communication, AI-driven translation tools such as Straive’s, are making a significant impact in the way pharmaceutical companies handle translations, ensuring accuracy, efficiency, and compliance.
Precise Translation is Crucial for Pharmaceutical and Life Sciences Success
There are several scenarios where translation is an essential part of the process. Here are some of them.
- Regulatory Compliance: Pharmaceutical companies must adhere to strict regulatory standards in scenarios such as launching new products in different markets, or conducting clinical trials or handling protected health information (PHI). Accurate translation of documentation, including clinical trial results, product labels, and patient information leaflets, is essential to meet these requirements. Errors or inconsistencies can lead to delays, fines, or even rejections from regulatory bodies.
- Patient Safety: Inaccurate translations can result in serious health risks, such as incorrect dosage instructions, misunderstood side effects, or improper medication usage. Ensuring that patient information is translated with precision is crucial for maintaining safety and trust.
- Global Collaboration: The pharmaceutical industry relies on international collaboration, with researchers, healthcare professionals, and regulatory agencies working across borders. Accurate translations facilitate communication, ensuring that all stakeholders are on the same page, whether it’s during clinical trials, research, or the distribution of products.
Types of Translation Approaches
There are two primary approaches to translation in the pharmaceutical industry:
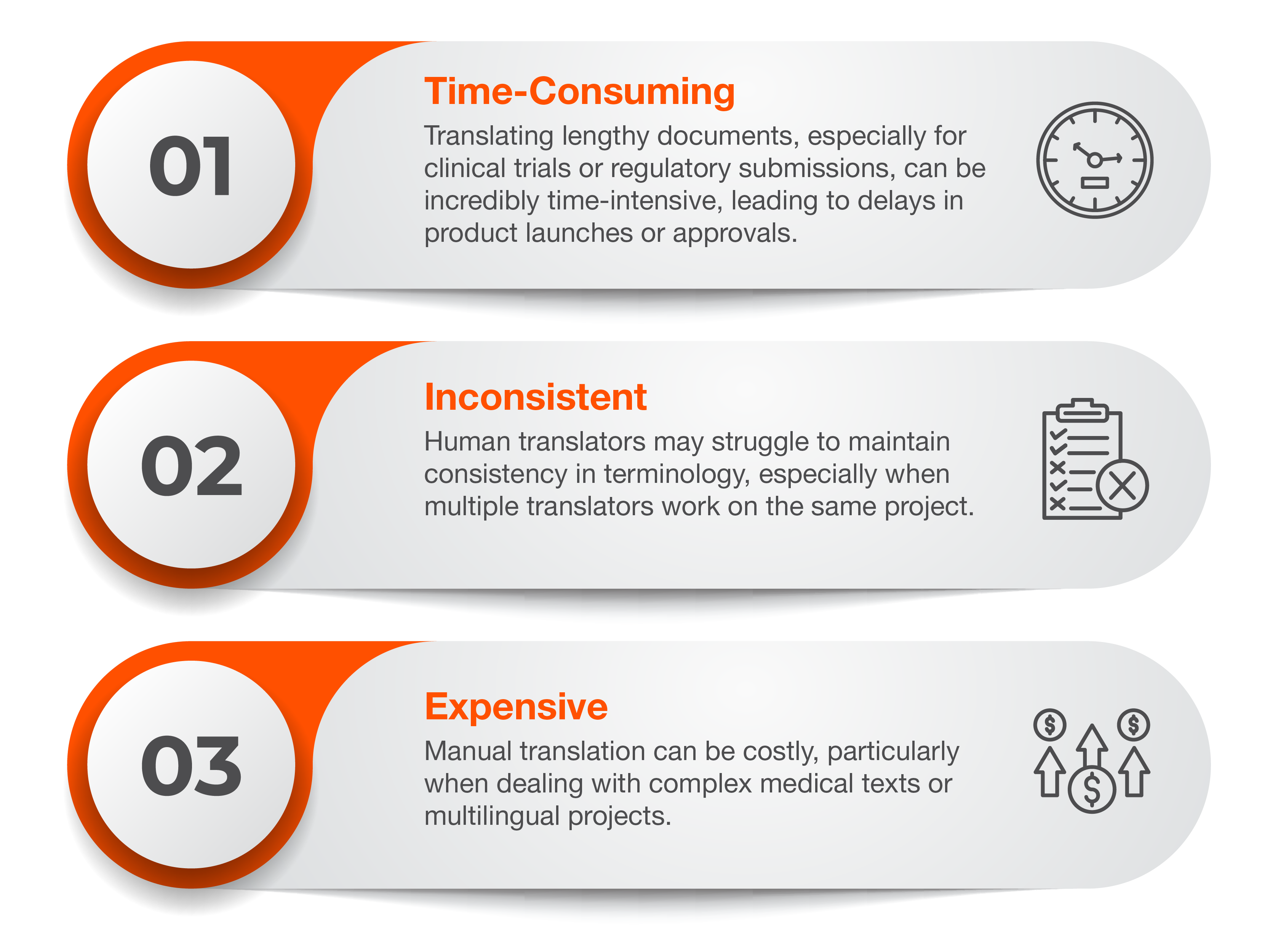
- Manual Translation: Traditional manual translation involves human translators with expertise in the pharmaceutical field. While it can offer high-quality results, it’s time-consuming, expensive, and prone to human error, especially when dealing with large volumes of text or highly specialized terminology.
- AI-Based Translation: AI-driven translation such as Neural Machine Translation (NMT), offer an efficient alternative. NMT tools leverage advanced algorithms to translate content quickly and accurately, handling large volumes of data and adapting to the specific terminologies of the pharmaceutical sector.
Challenges Faced in Manual Translation
While manual translation has been the standard for many years, it comes with its own set of challenges:
How Generative AI is Transforming Pharma Translations
Generative AI is transforming pharma translation by improving accuracy, speed, and cost-efficiency in handling complex medical and pharmaceutical texts. But how is it enabling this? Here’s how:
Generative AI is enhancing translation accuracy and consistency
- Medical and Regulatory Terminology: Generative AI models are trained on vast datasets of medical and regulatory texts, allowing them to better understand specific jargon and provide more accurate translations.
- Consistency Across Documents: Pharma requires consistency in terms like drug names, dosages, and clinical terminology. AI ensures consistent translations across a range of materials, from clinical trial reports to patient-facing documents.
Generative AI is ensuring faster translation turnaround
- Real-Time Translations: Generative AI can translate large volumes of content quickly, reducing the time required for cross-border drug approvals, clinical trials, and regulatory submissions.
- Support for Multilingual Documents: As pharma is highly globalized, AI allows for the simultaneous translation of documents into multiple languages, cutting down the time for market entry in different countries.
Generative AI improves compliance and risk management
- Regulatory Compliance: Even minor translation errors can lead to significant compliance risks. AI models can be fine-tuned to recognize and adhere to regulatory standards in specific regions, helping to minimize risk.
- Auditability: AI models used for translation provide detailed logs of changes and decisions, creating transparency in the translation process, which is crucial for regulatory submissions and audits.
Generative AI can improve cost efficiency
- Reduced Human Dependency: While human oversight is still required, AI can handle the bulk of translation work, reducing reliance on human translators and lowering costs.
- Scaling Up : AI can handle large-scale translation projects without the cost increasing in proportion to the volume, making it ideal for large multinational pharmaceutical companies operating globally.
Generative AI allows patient-centered translation
- Localized Information for Patients: Generative AI can customize translations to local dialects and cultural nuances, improving patient comprehension of drug instructions, informed consent documents, and clinical trial information.
- Automated Translation of Medical Literature: AI can translate medical research papers, treatment protocols, and clinical trial results, making them more accessible to healthcare providers worldwide.
Generative AI bolsters real-time multilingual communication
- Clinical Trials: AI-driven translation can enable faster communication between trial sites, especially in multinational trials, ensuring real-time updates and compliance with local regulatory requirements.
- Digital Health Applications: Many pharma companies are leveraging AI-driven translation for mobile apps and patient portals, allowing for real-time interaction in different languages.
Generative AI is helping the pharma industry streamline its processes, adhere to regulatory requirements, and reach patients in multiple languages faster and more efficiently. However, human oversight remains critical to ensure the accuracy of translations in such a highly regulated and safety-critical industry.
Straive’s AI-Powered Translation
Straive’s AI-powered pharmaceutical translation offers cutting-edge solutions tailored to the complex needs of the pharmaceutical and life sciences industry. Leveraging advanced natural language processing (NLP) models and machine learning, our translation solutions ensure accurate, context-aware translations of clinical trials, research papers, regulatory documents, and patient information across multiple languages.
With a focus on maintaining regulatory compliance, it streamlines the localization process while ensuring precision in pharmaceutical terminology and language nuances. By integrating with other AI-driven platforms like regulatory systems, clinical trial management, and customer support tools, Straive enhances global communication, enabling pharmaceutical companies to operate more efficiently across diverse markets.
Some key benefits of deploying Straive’s translation offerings are:
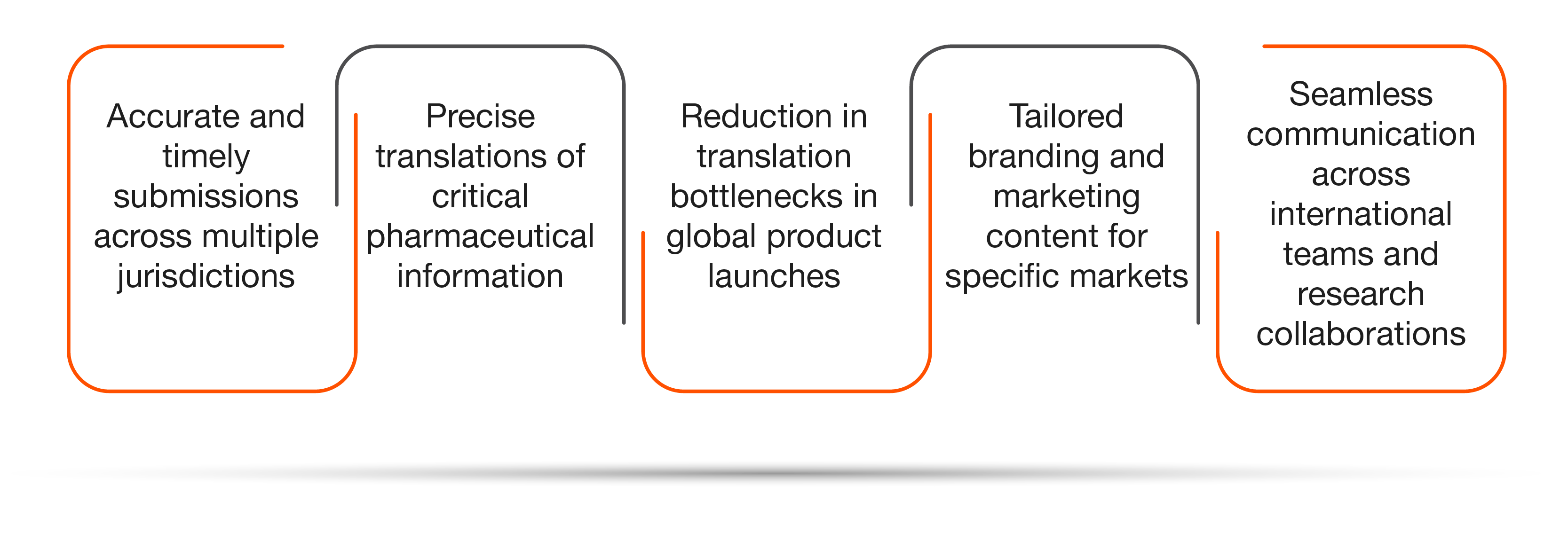
Our Deep Learning-driven translation engines combine machine translation with human oversight to support pharmaceutical companies in their content localization efforts.
A leading pharmaceutical company used our GenAI-driven solution to expedite the translation of pharma documents such as clinical trial documentation, research reports and more. By combining AI with human oversight, the company reduced translation time by 50% while ensuring compliance with regulatory standards.
Future View of AI Translation in Pharma
With Generative AI capabilities intertwined, translation in the pharmaceutical industry looks promising, with several more trends on the horizon:
Integration with Other AI Tools: AI translation tools will increasingly integrate with other AI-driven solutions, such as AI-based content management systems or regulatory compliance tools, creating a seamless workflow. For instance,
- Integration with NLP Tools for Drug Discovery and Research
AI translation tools can work alongside Natural Language Processing (NLP) models used in drug discovery, helping translate research papers, clinical trial documents, or patents from various languages. This would allow researchers across the globe to access and understand key findings and collaborate more easily. - Integration with Regulatory AI Systems
AI translation tools can integrate with regulatory AI systems that handle documentation and validation of compliance across regions.For example, automatically translating submission documents into the official languages required by the FDA, EMA, or other regulatory bodies, while ensuring consistency with regulatory AI systems that check for compliance issues. - Integration with AI for Clinical Trials
Pharmaceutical companies often run global clinical trials that require communication with diverse patient groups. AI translation tools can integrate with clinical trial management AI systems to ensure that patient consent forms, study protocols, and results are available in local languages. - Integration with AI Chatbots for Patient Support
Many pharmaceutical companies use AI-driven chatbots to provide support for patients, such as answering questions about medications or helping patients follow treatment regimens. AI translation tools can be integrated with these chatbots to ensure they can communicate with patients in multiple languages. - Integration with Machine Learning Models for Data Analysis
Pharmaceutical companies analyze large datasets from clinical trials, research, and patient outcomes. AI translation tools can be integrated into machine learning models that process multilingual data, ensuring that data from studies in different countries is consistently understood and analyzed. - Integration with AI for Medical Device and Drug Labeling
Drug and medical device labels need to be translated and adapted to local regulations. AI translation tools can integrate with labeling management AI systems that ensure accuracy and compliance across different markets. - Integration with AI for Knowledge Management
Pharma companies manage huge amounts of knowledge in the form of research, case studies, clinical trial results, and regulatory submissions. AI translation tools can be integrated into AI-based knowledge management platforms to ensure global teams have access to all necessary documents in their preferred languages.
Advancements in NMT: As NMT technology evolves, translations will become even more accurate, capable of handling nuanced and complex medical language.
Increased Regulatory Acceptance: As AI translation tools continue to improve, regulatory bodies are expected to become more accepting of AI-generated translations, along with regulations that can effectively control how AI is used by pharmaceutical and life sciences companies.
Conclusion
AI-driven approaches are increasingly becoming the preferred choice for pharma companies seeking to streamline their global communication efforts. By adopting best practices and combining AI with human expertise, pharmaceutical companies can ensure that their translations are accurate, consistent, and compliant, paving the way for a safer and more efficient industry.
At Straive, our translation solution offers cross-domain agility across multiple jurisdictions. If you’re a pharmaceutical and life sciences company looking to expand globally or seeking to improve your translation efforts,you can explore how we are using AI translation tools to enhance operations of global firms such as yours. Or, reach out to us for more information.
About the Author

Priyanka Mishra is a Manager of Creative Content at Gramener. Besides writing about Data Science, Priyanka loves reading about new concepts in marketing and emerging technology.
We want to hear from you
Leave a Message
Our solutioning team is eager to know about your
challenge and how we can help.